HOME > Program Members > Hideyuki Okano

Hideyuki Okano

Professor, Department of Physiology, Graduate School of Medicine, Keio University / Chairman, Graduate School of Medicine, Keio University
Hideyuki Okano, MD, PhD
hidokano@sc.itc.keio.ac.jp
http://www.okano-lab.com/
Theme
Neural stem cells (NSCs) possess the capability for self-renewal and are multipotent, that is, they can give rise to both neurons and glial cells. Embryonic NSCs are expected as a source for transplantation therapy, and adult NSCs as a basis of noninvasive treatment of neurological disorders. However, because the fate of embryonic NSCs is generally predetermined and restricted temporally, a given embryonic NSC cannot generate all of the cell types existing in the CNS. For example, early NSCs generate neurons but not glia; later NSCs generate both types of cells, however, they cannot give rise to early-born neurons. Meanwhile, actual use of adult NSCs for regenerative medicine is a vision far from being fulfilled because of the as-yet unexplored physiology and mechanisms of adult neurogenesis.To resolve these problems, we propose to establish in-vitro culture systems, and to develop a powerful model for investigating the mechanisms underlying early CNS development. Furthermore, we are examining adult neurogenesis both anatomically and physiologically by combining comprehensive in-vitro analyses, such as DNA microarray and ChIP-sequencing, and in-vivo analyses using knockout or knock-in mice. Findings from these approaches are expected to be applicable to regenerative therapy for neurodegenerative disorders.
Research activities
First of all, we established a simple ES cell culture system in which purified early NSCs were derived as neurospheres and their temporal identities were regulated in a single culture system (Okada Y et al. Stem Cells 2008). This in-vitro system recapitulates in-vivo CNS development, and therefore serves as a powerful model for investigating the mechanisms underlying early CNS development.Next, to identify the genes that are involved in regulating the initiation of the temporal change, we compared the global gene-expression profiles between the NSCs in the early and mature stages by DNA microarray. We then carried out functional screening of the genes that were expressed more strongly in the early-stage NSCs by lentivirus-mediated overexpression and knockdown. Among the genes examined, Coup-tfI/II knockdown resulted in the inhibition of acquisition of gliogenic competency by mature-stage NSCs, and in their prolonged generation of early-born neurons. In addition, the phenotype of Coup-tfI/II knockdown in the developing mouse brain was consistent with our in-vitro results. Thus, we identified COUP-TFI and II as key regulators required for proper temporal specification of NSCs, including their acquisition of gliogenic competency (Naka H et al. Nat Neurosci 2008).
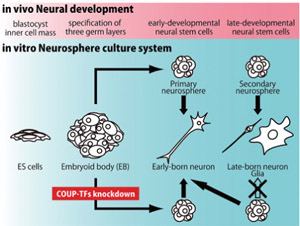
Fig.1
In-vitro culture system in which the temporal specification of neural development is recapitulated. Using this system, we showed that COUP-TFI/II are required for proper neurogenic-to-gliogenic transition of neural stem cells.
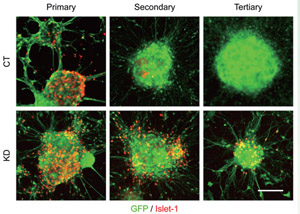
Fig.2
Coup-tfs knockdown extended the production period of Islet-1-positive motor neurons.
Selected Paper
- Naka H, Nakamura S, Shimazaki T, Okano H. Requirement for COUP-TFI and II in the temporal specification of neural stem cells in central nervous system development. Nature Neurosci. 2008 Aug 24:11 (9): 1014-1023.
- Kaneko S, Iwanami A, Nakamura M, Kishino A, Kikuchi K, Shibata S, Okano HJ, Ikegami T, Moriya A, Konishi O, Nakayama C, Kumagai K, Kimura T, Sato Y, Goshima Y, Taniguchi M, Ito M, He Z, Toyama Y, Okano H. A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat Med. 2006 Dec;12(12):1380-9.
- Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y, Okano H. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med. 2006 Jul;12(7):829-34.
- Nagoshi N, Shibata S, Kubota Y, Nakamura M, Nagai Y, Satoh E, Okada Y, Mabuchi Y, Katoh H, Okada S, Fukuda K, Suda T, Matsuzaki Y, Toyama Y, Okano H. Ontogeny and Multipotency of Neural Crest-Derived Stem Cells in Mouse Bone Marrow, Dorsal Root Ganglia and Whisker Pad.Cell Stem Cell 2. 2008 Apr 10:2(4):392-403.
- Sawamoto K, Wichterle H, Gonzalez-Perez O, Cholfin JA, Yamada M, Spassky N, Murcia NS, Garcia-Verdugo JM, Marin O, Rubenstein JL, Tessier-Lavigne M, Okano H, Alvarez-Buylla A. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006 Feb 3;311(5761):629-32.
Copyright © Keio University. All rights reserved.
